Covaxin Who Approval Status Latest News, Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog
WHO and an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and arrive at a decision on granting EUL to Covaxin. Why Hasnt Covaxin Been Approved Yet.
Covaxin Experts Fret Over Delay In Who Nod For Covaxin The Economic Times
What Can Possibly Explain WHOs Delay In Approving Covaxin.
Covaxin who approval status latest news. The countrys indigenous Covid-19 vaccine has not yet received emergency use approval from WHO. Vaccine triumphalism may have driven Covaxins approval and use in India. Acceptance of the manufacturers expression of interest EOI a pre-submission meeting between WHO and the manufacturer acceptance of the dossier for evaluation by WHO decision on status of assessment and final approval decision.
The process for getting Covaxins Emergency Use Listing EUL authorisation by the World Health Organisation WHO is proceeding very well Dr VK Paul member of NITI Aayog said on Monday. Hello Guys In this video we will know covaxin approval date by whoLink for article httpswwwbusinesstodayincoronavirusstorywho-approval-for-covaxin-. Come 5 October Indians who took two shots of Bharat Biotechs Covaxin will know if they have to wait longer before they can travel abroad.
The World Health Organisation will meet on Tuesday to discuss the long-awaited fate of Indias first homemade vaccineCovaxin has been used in India since its approval for emergency use in JanuaryIt accounts for about 11 per cent of total shots administered in the country. The World Health Organisation WHO is expected to grant emergency use authorisation to COVID-19 vaccine Covaxin soon but approval will likely be delayed. Bharat Biotech said on Friday that it has submitted all the data to WHO for emergency use listing EUL of Covaxin and is now awaiting feedback.
On Thursday the Central government announces that Indias Covaxin to receive WHOs approval any time soon after August 15. As Covaxin is yet to receive approval from the WHO it remains a concern for those who want to travel abroad. Why Covaxin is yet to win WHOs emergency approval Premium Earlier this week WHO in a series of tweets said it is waiting for one piece of information from Bharat Biotech to decide.
Covaxin who approval status latest news. This step would mean a big boost for the nationwide vaccination drive. He also stressed that in a few days India will achieve administration of 1.
WHO said after a meeting on Tuesday that its independent experts had sought additional clarifications from Covaxin maker Bharat Biotech. Get latest Covaxin Who Approval news updates stories. The latest Status of COVID-19 vaccines within WHO EULPQ evaluation process guidance document dated September 29 on the WHO website said that the decision date for Bharat Biotechs Covaxin is October 2021.
Bharat Biotech is likely to get approval from the World Health Organization in two weeks for the emergency use listing EUL of its Covaxin vaccine. Foreign Secretary Harsh Vardhan Shringla has said that Bharat Biotechs Covaxin Indias first indigenous Covid-19 vaccine will be approved by the World Health Organisation WHO soon. As Bharat Biotech had submitted the data required for WHOs approval on July 9 2021 the process is.
Amid the continued anticipation of WHOs emergency use approval for Indias indigenous vaccine made by Bharat Biotech - Covaxin a recent development suggests that a technical advisory group of the WHO scientists had been reviewing data produced by the vaccine manufacturers on Tuesday October 26 and are expected to make a call soon on the listing of Indias COVID-19 vaccine. Its manufacturer Bharat Biotech first submitted an expression of interest in applying to the WHO for emergency use in. The World Health Organisation WHO has updated the decision date for Bharat Biotechs Covaxin as October 2021 on its latest Emergency Use Listing EU.
Latest News India. Decision on Bharat Biotechs Covaxin EUL in October says WHO. By Bhaswati Guha Majumder - Oct 20 2021 0146 PM.
WHO further delays Covaxin clearance over technical queries Bharat Biotech has already completed the Phase 23 trials of Covaxin for use in children under 18 years of age. The WHO approval procedure for a vaccine consists of four steps. As of now only a handful of countries have confirmed that they will allow entry to those.
Even as Bharat Biotechs Covaxin got emergency approval from the WHO bringing relief to Indians on the eve of Diwali the agencys chief scientist Soumya Swaminathan has said that this will help speed up Covaxin Approval for children. Covaxin WHOs emergency use nod to Covaxin. All India Asian News International.
While the WHO has so far approved Covid-19 vaccines of Pfizer-BioNTech AstraZeneca-SK BioSerum Institute of India Johnson Johnson-Janssen Moderna and Sinopharm for emergency use Covaxins approval is awaited. Speaking to NDTV Swaminathan said In this case it should be much faster. But this might end up undermining Indias global pharmaceutical status says Aarti Betigeri.
A decision on Covaxins inclusion in the Emergency Use List will be taken in 2-3 months time the Union government informed the Rajya Sabha on Tuesday. The World Health Organizations WHO is yet to approve. The foreign secretary further stressed that its a technical process and not an administrative or a political process.

Bharat Biotech S Covaxin Likely To Get Who S Emergency Use Approval Today

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog

Who Says Decision On Covaxin S Emergency Use Listing Recommendation Expected Within 24 Hrs

Covaxin Approval Soon Who Chief Scientist Says Phase 3 Trial Data Looks Good Breaking News Youtube
Bharat Biotech S Covaxin Recognised By Australia For Travel India News India Tv

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod

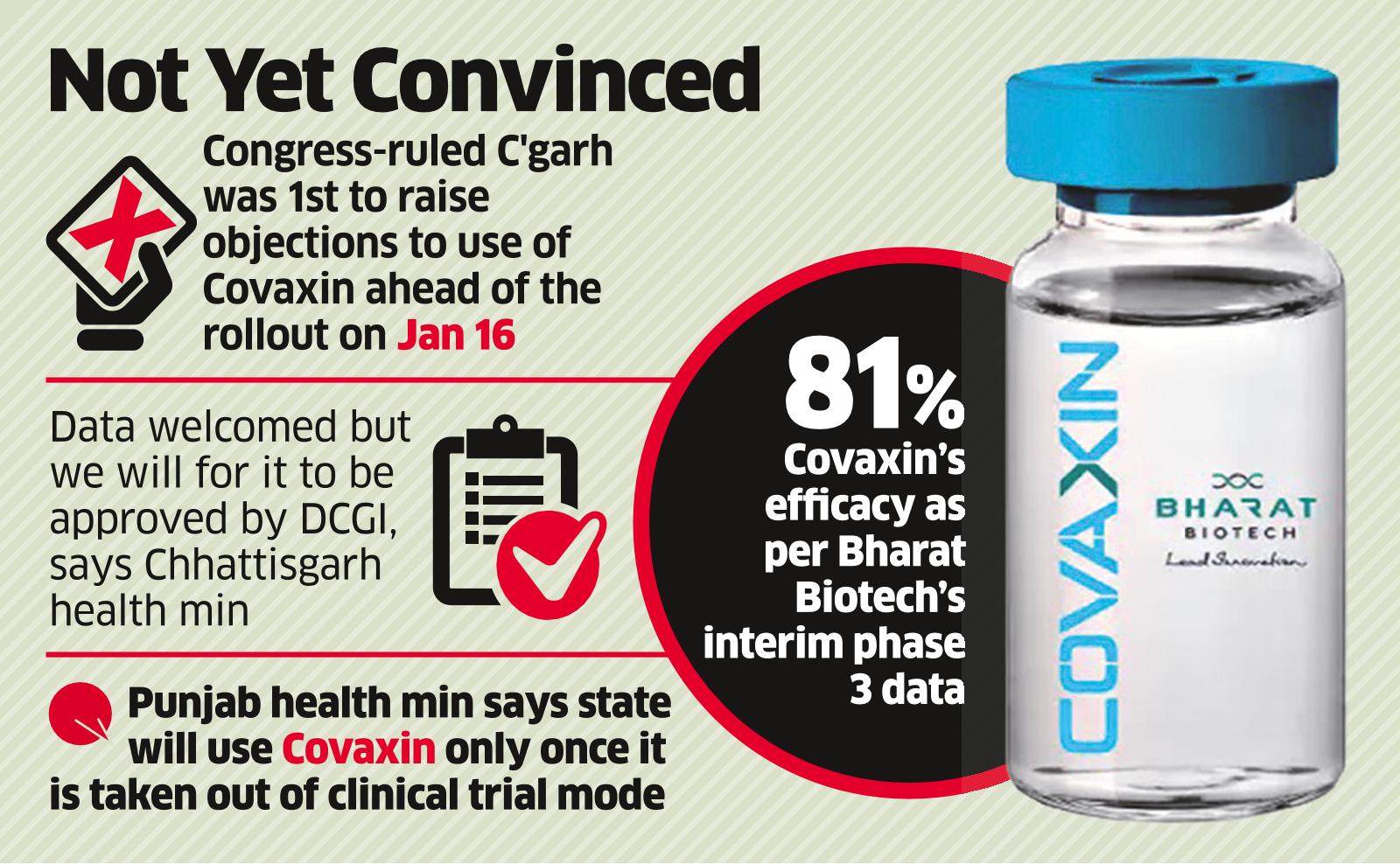
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times

Covaxin S Us Approval Delayed As Fda Asks For More Data India News Times Of India

Covaxin Has Not Got Emergency Use Approval From Who Since 6 Months Know The Process Here
Bharat Biotech S Covaxin Gets Who Approval For Emergency Use Listing Business Standard News
Covaxin S Lack Of Usfda Approval A Roadblock In Getting Who Green Flag The Hindu Businessline

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News
As Covaxin Awaits Who Approval Here S List Of Countries Where It Is Cleared Latest News India Hindustan Times

Who Seeks Additional Clarifications For Covaxin Approval Final Assessment Of Bharat Biotech S Covid Vaccine On November 3 India News
Bharat Biotech Submitting Data Regularly Very Quickly Who Official On Covaxin Approval India News Zee News