Covaxin Who Approval Date / Retjjanyoi2dem
On October 12 2021 Bharat Biotechs Covaxin got approved for usage on children between 2 to 18 years of age. The WHO has approved COVID-19 vaccines by Pfizer-BioNTech AstraZeneca Johnson and Johnson Moderna and Sinopharm till date.
The more products we have to fight COVID19 the better but we must keep up.

Covaxin who approval date. The vaccine was also approved for emergency use in Iran and Zimbabwe. Decision on Covaxin approval likely within 24 hours. The WHO said it began rolling data of the vaccine on 6 July.
The wait for Indias first indigenously-developed COVID-19 vaccine to receive World Health Organisation WHO approval is set to end within a day. An important decision from the WHO is fast approaching. Eager for WHO approval for Covaxin.
The country has also approved Covaxin. The African country has approved the made-in-India vaccine Covaxin. The latest news is the World Health Organization approval for the emergency use authorization EUA to Bharat Biotech-manufactured Covid-19 vaccine- Covaxin is likely to be delayed till October 5.
A WHO spokesperson said a technical advisory group was reviewing data on Covaxin and if all is in place and all goes well and if the. As countries open their borders to travel tourism and education Bharat Biotechs Covaxin awaits WHOs nod on October 26. Though Covaxin was licenced by Indian regulators in January this year when the company began phase 2 studies WHO requires evidence from the phase 3 trial.
Covaxin decision date to be confirmed says WHO Premium A health worker preparing a dose from a vial of Covaxin a Covid-19 vaccine. This approval of shelf-life extension is based on the availability of additional stability data which was submitted to CDSCO Bharat Biotech said in a tweet. On arrival tourists and non-residents with proof of a valid negative PCR test result should expect to be required to quarantine for 10 days.
Covaxin is still to be authorised by Western authorities. The WHO is currently reviewing the data submitted by the vaccine maker and the date for a decision on the jab is October 2021 according to the update available on the WHO website. As of date India is.
WHOs advisory group to meet on 26 Oct to decide on EUL for Covaxin says chief scientist Soumya Swaminathan The approval for the vaccine is much awaited especially by students medical tourists business travellers and people with international. According to the Indian embassy in Oman All passengers from India who have received two doses of Covaxin at least 14 days before the estimated arrival date will now be able to travel to Oman without the requirement of quarantine. It was approved for adults in India this January.
It was approved for adults in India this January. Collection of Covaxin who approval date The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. The latest Status of COVID-19 vaccines within the WHO EULPQ evaluation process guidance document dated 29 September on the WHO website said that the decision date for Bharat Biotechs Covaxin is October 2021.
Co-founder Suchitra Ella is hopeful of a positive answer. Hyderabad-based Bharat Biotech which has developed Covaxin had submitted EOI Expression of Interest to the World Health Organisation on. The burning question across India is when would the World Health Organization WHO grant Indias.
However the World Health Organisations approval for the emergency use authorisation EUA to Covid-19 vaccine Covaxin is likely to be delayed as per international public health agency. A WHO official has said the agencys assessment of made-in-India. A day after the US Food and Drug Administration FDA denied approval for emergency use of Covaxin the Hyderabad-based Bharat Biotech on Friday said that it is following the recommendation for additional data and will work for full approval.
The WHO has approved COVID-19 vaccines by Pfizer-BioNTech AstraZeneca Johnson and Johnson Moderna and Sinopharm till date. The CDSCO has approved the extension of shelf life of Covaxin up to 12 months from the date of manufacture. Glad to see one more vaccine Covaxin being granted WHO emergency use listing.
The WHO is currently reviewing the data submitted by the vaccine maker and the date for a decision on the jab is. OCGN Stock Soars 30 as WHO Kicks Off COVAXIN Review. This emergency approval granted without considering Phase III trial data concerning efficacy and safety drew widespread criticism.
The process will extend Covaxin timelines for seeking US approvals the company said in a statement. The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. WHOs advisory group set to meet on October 26 to decide on Covaxin EUL approval.
Aug 12 2021 Updated Aug 12 2021 953 AM IST. WHO to Take Final Decision Next Week on Approval to Bharat Biotechs Covaxin.

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog

Bharat Biotech S Covaxin Likely To Get Who S Emergency Use Approval Today
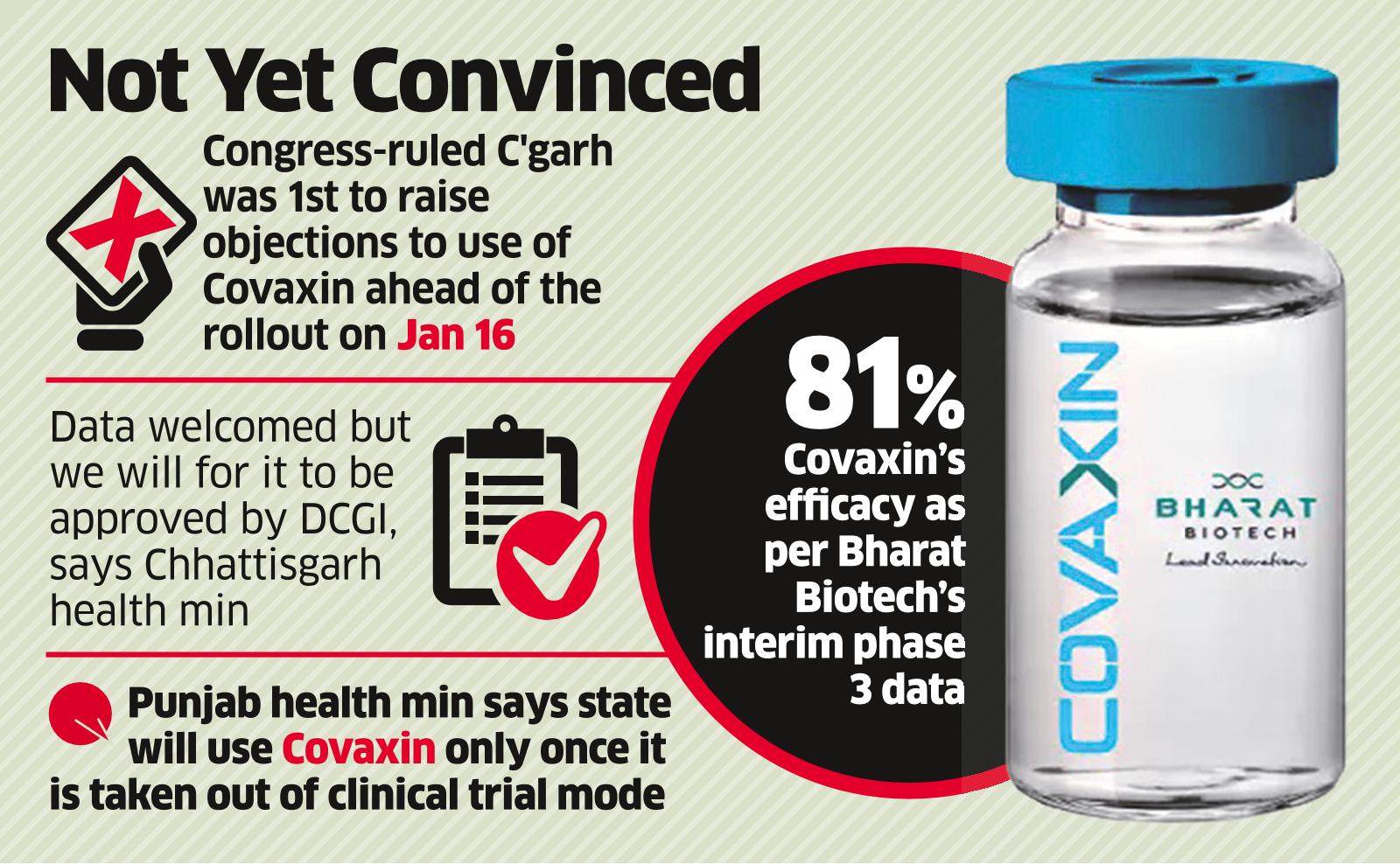
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times

India Gets 2 Vaccines Against Coronavirus As Covaxin Covishield Get Final Nod By Dcgi

Crucial Who Meeting On Bharat Biotech S Covaxin Approval Concludes Breaking News Youtube
Covaxin Shelf Life Increased To 12 Months From Date Of Manufacture
Submitted All Documents To Who For Emergency Use Listing Of Covaxin As Of July 9 Bharat Biotech Latest News India Hindustan Times

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod

Bharat Biotech S Covaxin Gets Shelf Life Extension Up To 12 Months From Date Of Manufacture

Bharat Biotech S Covaxin Gets Who Approval For Emergency Use Listing Business Standard News

Difficult To Predict Time Frame But Covaxin Will Get Who S Approval Says Aiims Professor

Who Delays Covaxin Approval Seeks Additional Clarifications From Bharat Biotech India News India Tv

Covaxin May Soon Get Who Approval As Expert Group Scheduled To Meet Next Month

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News

Covaxin Has Not Got Emergency Use Approval From Who Since 6 Months Know The Process Here


)
